First Michigan patient receives gene therapy for hemophilia A outside clinical trials
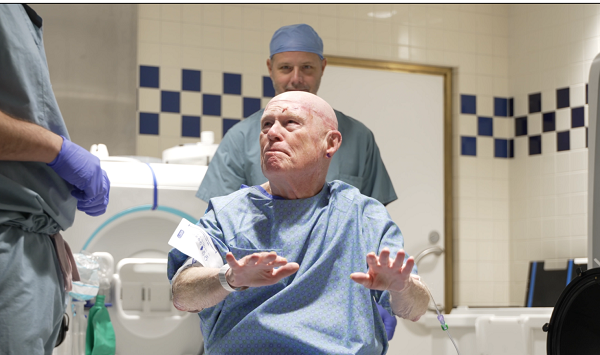
DETROIT — A Michigan man in his early 30s has become the first patient in the state outside of clinical trials to receive a cutting-edge gene therapy for hemophilia A, an inherited condition that often requires lifelong infusions to prevent bleeding episodes.
The patient, who has a severe form of hemophilia A, received an infusion of valoctocogene roxaparvovec-rvox (Roctavian) on Jan. 15, 2026, at the Infusion and Chemotherapy Treatment Center at Henry Ford Cancer – Detroit. His care was overseen by Dr. Philip Kuriakose, chief of hematology at Henry Ford Hospital and medical director of the system’s Hemophilia and Thrombosis Treatment Center.
“Living with hemophilia means planning much of your day-to-day life around the risk of bleeding,” said Dr. Kuriakose. “Through gene therapy, we can give patients a working gene to replace the faulty one, allowing their bodies to function more like they would without the condition. For many patients, that kind of freedom — both from regular at-home treatments and from constant worry — is something they’ve never experienced before.”
An estimated 33,000 males in the U.S. are living with hemophilia. Hemophilia A, the most common form, affects about 12 out of every 100,000 males in the country. People with hemophilia A do not produce sufficient levels of factor VIII, a protein essential for blood clotting. That puts them at risk for bleeding from minor injuries, frequent bruising, spontaneous internal bleeding and painful bleeding into the muscles and joints.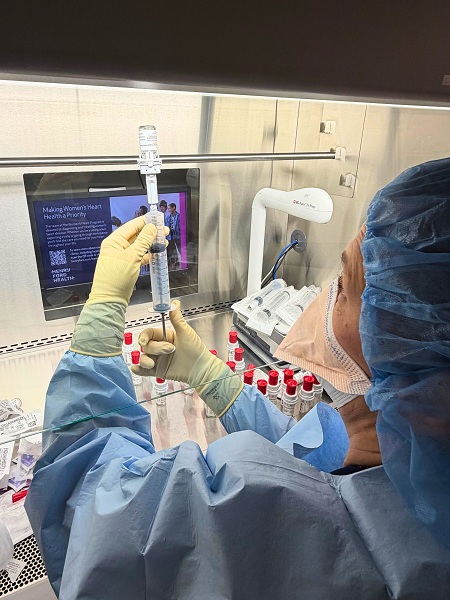
Many manage the condition through at-home injections or infusions. That can mean establishing an IV and injecting replacement clotting factor sometimes several times per week. The cost is also substantial, with replacement clotting factor averaging about $300,000 per year for patients with severe hemophilia, according to the National Bleeding Disorders Foundation.
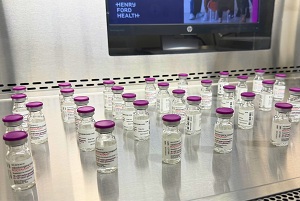
Roctavian, the first FDA-approved gene therapy for adults with severe hemophilia A, is a one-time infusion that delivers a functional copy of the factor VIII gene, instructing the liver to produce the clotting protein on its own. Studies show it can significantly reduce bleeding episodes and, in many cases, eliminate the need for routine home infusions or injections. At the end of a five-year clinical trial, more than 80% of participants did not need regular at-home factor replacement.
“We start caring for many hemophilia patients when they are children and see first-hand how much time, energy and money it takes to manage this condition," said Laura Gusba, a nurse practitioner in Henry Ford Health’s Hemophilia and Thrombosis Treatment Center. “Just imagine being able to live your life without having to plan around home infusions and constantly taking steps to minimize the risk of bleeding."
In addition to being the first commercial use of Roctavian in Michigan, the recent infusion marked the first time that Henry Ford Health has administered IV gene therapy outside of clinical trials — a significant milestone for the system’s growing gene therapy program.
As interest in gene therapy continues to rise, leaders across the system have developed an infrastructure to support the delivery of these complex and expensive treatments, many of which cost millions of dollars per dose. That effort included hiring a dedicated pharmacist to oversee the safe, efficient and financially responsible administration of these therapies.
“Gene therapy can be life changing for patients, but it poses significant logistical and financial challenges for health systems,” said Justin Reid, a cell and gene therapy pharmacy specialist at Henry Ford Health. “Administering an infusion like Roctavian takes extensive coordination between our teams, drug manufacturers, insurance providers and other stakeholders. We’re thrilled that we have a blueprint for making this therapy available to patients across the Midwest.”
###
MEDIA CONTACT: mediarelations@hfhs.org
.svg?iar=0&hash=F6049510E33E4E6D8196C26CCC0A64A4)

/hfh-logo-main--white.svg?iar=0&hash=ED491CBFADFB7670FAE94559C98D7798)